Abstract
Background Andexanet alfa (AnXa; Andexxa) was developed as a specific antidote for direct and indirect factor Xa (FXa) inhibitors. It has been approved in the US for the reversal of apixaban and rivaroxaban anticoagulation when necessary due to life-threatening or uncontrolled bleeding. Generation 2 manufacturing process of AnXa is promising for providing adequate global supply of AnXa and awaits comprehensive regulatory evaluation for comparability to the current commercial process.
Purpose: In this ongoing porcine polytrauma study, we investigated and compared efficacy and safety of the first and second generation AnXa product for the reversal of apixaban anticoagulation.
Method: In 35 anesthetized male pigs, a polytrauma consisting of bilateral femur fractures and a standardized blunt liver injury was inflicted after 3 days of apixaban intake (20 mg/day). 12 min after the polytrauma, pigs were randomly allocated to receive (n=7 per group) either 1000 mg bolus of 2nd generation AnXa followed by 2-hour (h) infusion of normal saline (2nd G AnXa B) or 1000 mg bolus immediately followed by infusion of 1200 mg 2nd generation AnXa in 2 h (2nd G AnXa B+). These two groups that received 2nd generation AnXa were compared with the cohorts from our previous study following precisely the same protocol with bolus and 2 h infusion of normal saline (Control group), 1000 mg bolus of 1st generation AnXa followed by 2 h infusion of normal saline (1st G AnXa B), and 1000 mg bolus immediately followed by 2 h 1200 mg infusion of 1st generation AnXa (1st G AnXa B+). Animals were then observed for 5 h or until death. The blood loss (BL) was the primary outcome. Hemodynamics and coagulation profile comprised apixaban (anti FXa activity and rotational thromboelastometry (ROTEM). Further D-dimer levels and thrombin generation (TG) were measured. Right after the premature death or at the end of the observation period, internal organs were obtained and examined for the occurrence of thromboembolic incidents. Two-way ANOVA (mean ± SD) and log-rank tests were performed to analyze the data, and the statistical significance was set at p <0.05.
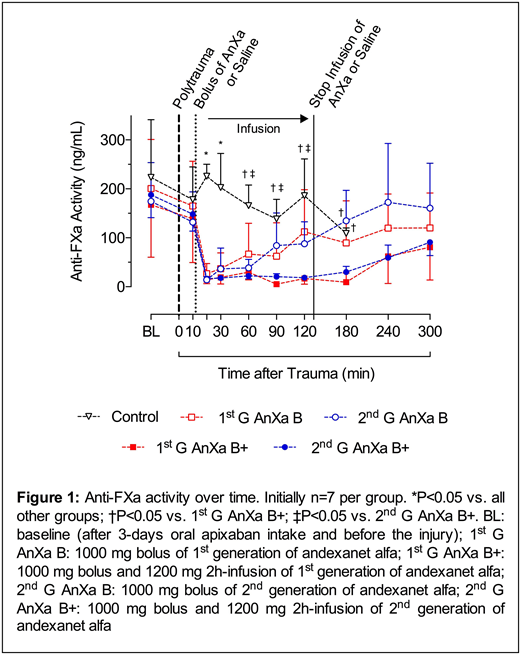
Results: Three days of oral apixaban administration resulted in comparable anti-FXa activity levels before the trauma (195.4 ± 90.9 ng/mL, Figure 1). The increased anti-FXa activity also prolonged the clotting time (CT) on extrinsically activated ROTEM channel EXTEM (43.6 ± 5.5 to 110.1 ± 28.2 sec). BL at 12 min after the trauma was similar among the study groups (856 ± 118 mL). Anticoagulation with apixaban in control group resulted in a total BL of 3403 ± 766 mL (p<0.01 vs. all other groups) with 100 % mortality (survival time: 92-193 min; p<0.01 vs. all other groups), whereas all animals treated with AnXa survived. Both regimens with 2nd generation AnXa (2nd G AnXa B and 2nd G AnXa B+) resulted in a significant reduction in total BL (1148 ± 58 mL and 1116 ± 128 mL, respectively). The decrease in total BL was similar to both therapy regimes of previous cohorts of 1st G AnXa B and 1st G AnXa B+ (1264 ± 205 and 1202 ± 95 mL, respectively). All AnXa treatments similarly reversed anti-FXa activity more than 90% (19.8 ± 15 ng/mL) within 5 min after the bolus. Similarly, in both bolus regimens (1st and 2nd G AnXa B) 30 and 56% of the initial anti-FXa activity respectively re-emerged at 60 and 120 min after the trauma due to the short half-life of AnXa (~1.6 h) and continued absorptionof apixaban. With the infusion regimens (1st G and 2nd G AnXa B+), 34 and 48% reappearance of anti-FXa activity were postponed to 240 and 300 min after the trauma, respectively. However, the return of FXa inhibition had no influence on total BL post trauma. AnXa restored EXTEM CT (45.3 ± 6 sec) within 5 min corresponding to the neutralization of apixaban. With the gradual reemergence of anti-FXa activity, EXTEM CT values were also prolonged. Compared to the control group, D-dimer levels increased significantly after the treatment with AnXa indicating activation of coagulation and in line with the restoration of impaired TG induced by apixaban. Neither clinical observations nor histopathologic examinations showed any sign of hypercoagulopathy.
Conclusion: This animal polytrauma model is the first to show that 2nd generation AnXa is as effective and well tolerated as the 1st generation in reducing blood loss and improving survival in apixaban anticoagulated pigs with massive hemorrhage.
Grottke:Boehringer Ingelheim: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding; Portola: Consultancy, Research Funding; Octapharma: Consultancy, Research Funding; Sanofi: Honoraria; Pfizer: Honoraria. Conley:Portola: Employment. Rossaint:CSL Behring: Honoraria; Boehringer Ingelheim: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal